Bioanalysis
End-to-end Bioanalysis and DMPK services
for faster drug development

How can integrated GLP and GCP-compliant bioanalysis accelerate drug development?
Fragmented bioanalysis can delay drug development and increase risk. Accelerate your drug discovery and clinical programs with integrated, GLP/GCP-compliant bioanalysis and DMPK services. From early-stage discovery to regulatory trials, Concept Life Sciences delivers high-quality, regulatory-ready data to help you make faster, confident decisions.
Supporting drug discovery to clinical trials
Gain confidence in every stage of drug development. Our integrated approach combines bioanalysis, DMPK, ADME/Toxicology and biomarker services under one roof, providing:
- Regulatory-ready results for GLP and GCP studies
- Streamlined workflows to reduce delays and lab transfers
- Actionable insights into pharmacokinetics (PK), pharmacodynamics (PD) and biomarkers
Benefit: Faster timelines, consistent data, and informed decision-making at every stage of your program.
Speak to an expert about your bioanalysis project.
Bioanalysis capabilities
We offer flexible, tiered bioanalytical services tailored to your molecule and project stage:
Sample analysis
GLP/GCP compliant sample analysis: Audited and compliant with industry regulations and guidelines.
Time-sensitive analysis: Rapid turnaround times to answer clinical questions without compromising data quality and patient safety.
Accurate PK data: Quantify drugs and metabolites throughout treatment regimens to assess drug performance and inform development decisions.
Sensitive biomarker detection: Detect subtle yet meaningful changes in endogenous biomarkers, critical for evaluating treatment effects or distinguishing disease states.
Method development and validation
Customized methods: for challenging or novel compounds and complex matrices.
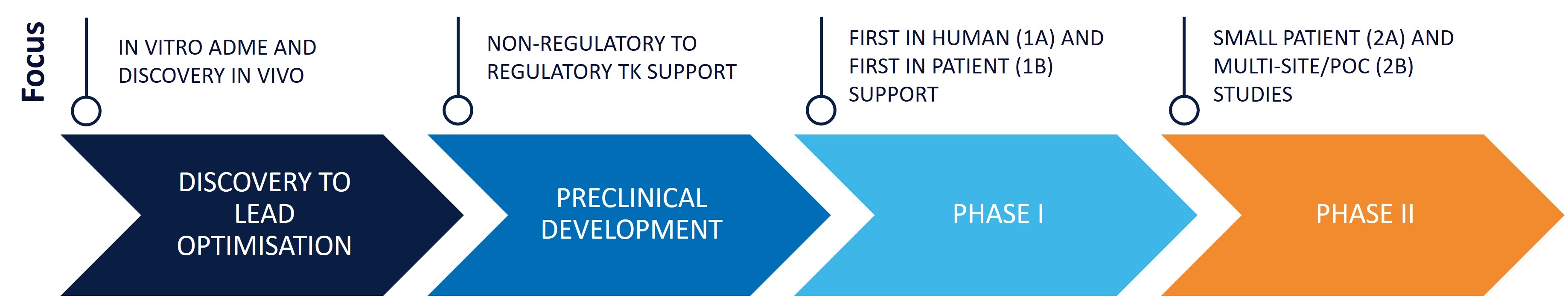
Seamless transition: from preclinical studies into successful Phase I and II trials.
Full validation: according to industry regulatory guidelines (ICH M10/FDA/EMA).
Non-clinical and discovery bioanalysis
For discovery and non-clinical programs, we deliver:
Rapid screening: Generate data quickly to guide compound selection and optimization.
Tailored method development: Robust data generation utilising generic methodologies through to bespoke compound and matrix specific optimisation.
Fit-for-purpose bioanalysis: R&D or scientific validation for in vitro projects and early-stage non-regulated/non-clinical analyses.
GLP-compliant regulatory analysis: Supporting pivotal preclinical trials.
Tiered service offering

Ideal For: Non-clinical GLP and clinical GCP studies
Turnaround: Per study schedule
GLP/GCP-compliant methods and QA-audited sample analysis for pivotal clinical trials supports non-clinical GLP and clinical GCP projects, conducting validations per industry guidelines with separate method development, validation, and analysis studies, including QA audits, long-term stability assessments, and incurred sample reanalysis (ISR).
Purpose / Key Benefits: Scientific validation for non-regulatory, early-phase or biomarker studies. Optional QA audits.
Ideal For: Early stage, non-clinical, biomarker studies or in vitro projects
Turnaround: 3-4 months
Scientific validation supports non-regulatory, early-phase, non-clinical/clinical and biomarker studies, and in vitro projects. Offers method validation in addition to standard services, with distinct studies for development, validation and sample analysis, with optional QA auditing.
Purpose / Key Benefits: Fully customized method development for novel or challenging compounds.
Ideal For: Unique compounds requiring extensive method optimization
Turnaround: Up to 6 weeks
Fully customized method development for challenging or novel compounds. A customized bioanalytical service for clients needing tailored methods and willing to invest time and resources into method development and robustness testing.
Purpose / Key Benefits: Adds method development to enhance specificity and robustness. Prioritizes speed, high-quality results.
Ideal For: More complex discovery projects needing tailored analysis
Turnaround: 1–2 weeks
Adds method development to enhance specificity and robustness. Enhanced bioanalysis with method development for your discovery projects, prioritising specificity, speed, and high-quality results.
Purpose / Key Benefits: Rapid, generic bioanalysis for early decision-making. Entry-level, robust data generation using proven methodologies.
Ideal For: Simple compounds and follow-up screens
Turnaround: Days
Fast, generic bioanalysis for early decision-making. Entry-level rapid and robust data generation utilising proven methodologies, ideal following on from screens or for simple, generic compounds.
Purpose / Key Benefits: Cost-effective optimization and compatibility testing. Uses minimal material and reduces animal usage.
Ideal For: Early-stage discovery and compound screening
Turnaround: Days
A cost-effective option for optimisation and compatibility testing of your compound, using minimal material and reducing animal usage.
Why Choose Concept Life Sciences for bioanalysis?
Transparency for end-to-end bioanalysis, from early-stage method development to GLP/GCP compliant sample analysis, we deliver:
Deep expertise: Across small molecules, peptides, oligonucleotides, biotherapeutics and immunology, immuno-oncology and neuroscience.
Flexible analytical capabilities: Accelerate preclinical drug development with LC-MS/MS analysis, for high sensitivity drug, metabolite, and biomarker quantification, with complementary HPLC-UV and GC-MS techniques available.
Regulatory-compliant bioanalysis services for clinical trials: GLP accredited laboratory with GCP capability.
Scientist-to-scientist collaboration: Strategic input, consultation and clear data interpretation.
Integrated services: Bioanalysis combined with ADME / DMPK, toxicology, chemistry and biology under one roof.
The result: Faster timelines, consistent data and informed decision-making, without losing scientific oversight.
Flexible, tiered bioanalytical services
Your molecule is unique and so is every project, your bioanalytical service should be too.
Our multi-tiered bioanalytical services provide exactly what you need, when you need it, whether rapid screening, custom method development, or fully validated regulatory testing; all under one roof.
Why our flexible tiered system works
- Cost control: Only pay for what you need, when you need it.
- Speed: Rapid screening and discovery options speed up decision-making.
- Continuity: Single partner for discovery through GLP/GCP.
- Customizability: Flexible solutions for start-ups, multi-site teams, and large pharma.
Bioanalysis FAQs
Q: What bioanalytical services does Concept Life Sciences provide?
A: We offer bioanalytical method development, PK screening, scientific and regulatory-level validation, and integrated in-life and biomarker sample analysis.
Q: What is GLP/GCP-compliant bioanalysis?
A: GLP/GCP-compliant bioanalysis ensures that sample analyses meet regulatory standards for clinical and non-clinical studies.
Q: How are bioanalysis services tiered?
A: Our flexible tiers range from rapid Discovery screening, Discovery+ method development, Discovery+ Bespoke custom methods, Scientific Validation for early studies, to Full Validation for GLP/GCP-compliant clinical trials.
Q: How quickly can I receive results?
A: Turnaround times vary by tier, but Discovery and PK Screening options provide rapid preliminary data within days, while full validation studies are scheduled per regulatory requirements.
Q: Can I combine bioanalysis with other services?
A: Yes — our integrated approach allows combination with in-life studies, biomarkers, and toxicology support for end-to-end project management.
