Neuroscience
Driving CNS drug discovery with advanced in vitro models
.jpg)
How can we help you unlock translational success in neuroscience drug discovery?
Expertise in a broad spectrum of neuroscience assays is difficult to find. Our team of expert neuroscientists pioneered translational research assays in neuroinflammation, myelination and neuronal biology. Collaborating with you to meticulously craft customized assays that exactly meet your project needs is our specialty, providing solutions to the most complex challenges. Our cutting-edge technologies, comprehensive quantitative readouts, and consultative approach will provide you with the high-quality data that you need to confidently progress toward the clinic.
Pioneers in neuroscience research services
Formerly Aquila Biomedical, we pioneered translational neuroscience assays and continue to support clients in developing breakthrough CNS therapies.
We understand that neuroscience is one of the most complex therapeutic areas. Developing therapies to address longstanding challenges, such as neurodegeneration, demands deep expertise and specialized solutions to translate research into real-world impact.
To support your journey through the discovery and preclinical development of novel CNS therapeutics, we harness in vitro and ex vivo models of increasing physiological relevance and complexity to craft unique assays. For novel challenges, we design bespoke approaches grounded in decades of neurobiological expertise.
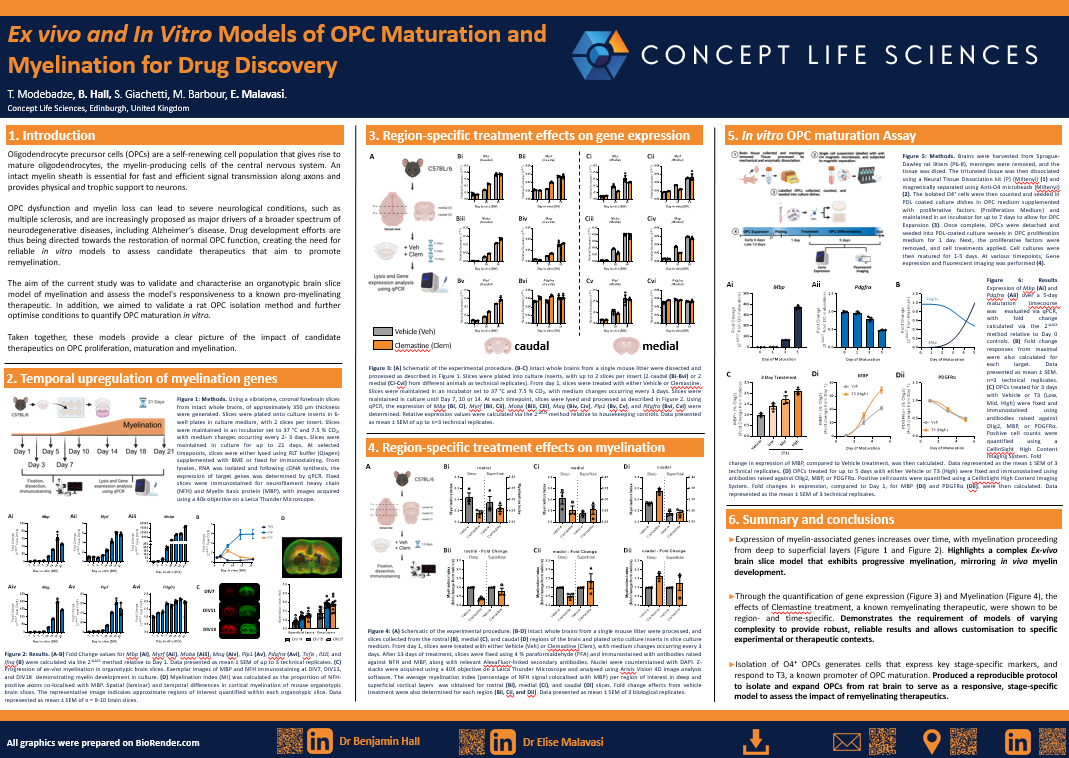
The three pillars of our expertise to drive neuroscience drug discovery programs
Our team of neuroscientists have deep expertise in:
- Neuroscience
- Myelin Biology
- Neuronal Biology
By seamlessly combining rodent and human iPSC-derived models, we deliver robust pre-clinical data for your drug discovery program. Explore our models here.
Neuroinflammation
Neuroinflammation is a central feature of neurodegenerative diseases. Multiple astrocytes and microglia signaling pathways become chronically dysregulated, triggering excessive and continued release of pro-inflammatory cytokines, chemokines, and neurotoxic molecules, contributing to neuronal damage and degeneration.
Modulation of these inflammatory pathways is a promising strategy for the treatment of neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease.
Are you looking for ready-made solutions or bespoke experimental designs? Our neuroscientists will be able to support your goals.
- Microglial polarization
- Astrocyte polarization
- Brain slice inflammatory activation
- Brain slice NLRP3 inflammasome activation
Myelin biology
The maintenance of healthy myelin is essential for fast and efficient conduction of action potentials along axons. Oligodendrocyte precursor cells (OPCs) are a self-renewing cell population that gives rise to mature oligodendrocytes, the myelin-producing cells of the central nervous system.
OPC dysfunction and myelin loss can lead to severe neurological conditions, such as multiple sclerosis, and contribute to a broader spectrum of neurodegenerative conditions, including Alzheimer’s disease.
Restoration of normal OPC and myelin function is a key target for neurotherapeutics. In the presence or absence of candidate therapeutics, we can quantify:
- OPC proliferation
- OPC maturation
- Brain slice myelination
Neuronal biology
The aim of any candidate CNS drug is to improve neuronal function and signaling pathways. In vitro neuronal models offer a controlled and reproducible system to study the effects of candidate drugs.
Neuronal monocultures are ideal to investigate the impact of a drug on cell-autonomous functions and pathways, or to reveal its potential neurotoxic or neuroprotective effects.
In contrast, neuron–glia co-cultures enable assessment of drug candidates that act indirectly on neurons by modulating glial activity or regulating neuron–glia communication. These co-culture systems offer a more physiologically relevant environment for evaluating neuroactive compounds and their mechanisms of action.
Thanks to our expertise in neurobiology and neurophysiology, we can guide you towards the most appropriate cell model, experimental design and readout to provide you with the answers you need.
Our expertise includes:
- Quantification of neurotoxicity and neuroprotection
- Kinetic assessment of neurite development in live neurons
- In-depth analysis of neurite network and synaptic density by High Content Imaging
- Measurement of organelle health and function such as mitochindrial health or autophagosome function
- Assesment of neuronal function by calcium flux
Support from concept to clinic
Our specialist neuroscience expertise is complemented by a wider offering across chemistry, ADMET, and GMP manufacturing services. Speak to our team to explore how we can help you bring your immunology therapeutic to the clinic.