- CD4+ and CD8+ activation assays
- Mixed Lymphocyte Reaction (MLR)
- Th1, Th2, Th17 and iTreg differentiation assays
- Exhaustion assays
- Treg functional assays
- Tumor killing assays (2D/3D models)
- Cytokine profiling
Immunology
Expert immunology research solutions for drug discovery

Our specialist Immunology team combines decades of experience in immunology with advanced human cell models to provide physiologically relevant insights that accelerate your drug discovery. With additional services across chemistry, ADMET and GMP manufacturing, we are able to provide seamless support across the drug discovery and development pipeline.
Leaders in immunology research services
Immunology is inherently complex, demanding deep expertise and specialized solutions to translate research into real-world impact. Our expert team delivers high-quality data and insight to empower decision-making throughout drug discovery and development. By using advanced human immune cell models, we generate physiologically relevant data that enhances the translational success of your program.
With a legacy spanning over 15 years, we pioneered translational immunology assays as Aquila Biomedical, and since then have continued to support clients as they bring cutting-edge immunology therapies to the clinic.
With our flexible and consultative approach, we work closely with you to provide custom-built solutions that are led by your goals, timelines and internal processes to deliver the insight needed to drive your project to the next stage.
Our suite of assays include:
- Activation and proliferation
- Antibody production
- ADCC (Antibody Dependent Cellular Cytotoxicity)
- Cytotoxicity assays
- Tumor killing assays
- Maturation and cytokine profiling
- One-way MLR
- Activation and cytokine profiling
- Polarization (M1/M2)
- Phagocytosis
- ADCP (Antibody Dependent Cellular Phagocytosis)
- Suppression Assays
- Inflammasome Assays
- Tumor killing (immune cell mediated)
- TAM (Tumor-Associated Macrophages)
- MDSC (Myeloid Derived Suppressor Cells)
- Broad immune profiling
- Cytokine multiplex assays
- Cytokines, immunoglobulins
- Immune cell phenotyping, receptor expression
- Gene expression
Reduce translational risk with advanced pre-clinical models that mirror patient biology
Human models are essential to more accurately predict human immune responses and clinical efficacy. Our team of immunologists has developed robust in vitro assays to recapitulate many key pathological processes relevant to the immunotherapy field. Our assays use human primary immune cells for the closest representation of clinical settings, delivering the most meaningful pre-clinical data for your program of work.
We have significant experience working with human primary immune cells, either whole PBMC, isolated T cells or other immune cells to assess compound effects in monoculture or co-culture assay systems, 2D and 3D spheroid models, and across many different modalities.
In addition, we offer readout to support your objectives, including comprehensive high-dimensional readout such as transcriptomics, high-content imaging and multiplex assays.
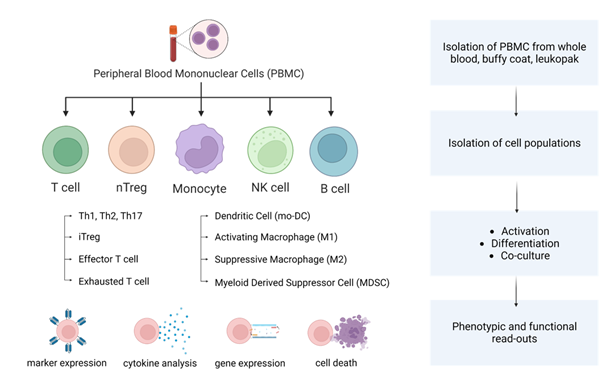
Optimized assays for clinically relevant insight
PBMC assays
Versatile assay for broad immune profiling
Peripheral Blood Mononuclear Cell (PBMC) assays provide a practical and informative approach for studying the effects of compounds across multiple immune cell types in a biologically relevant context. Widely used throughout the drug discovery process, from early-stage research to target validation and lead optimization, these assays help characterize both stimulatory and inhibitory responses.
- Capture compound activity across a diverse mix of immune cells, including T cells, B cells, monocytes, and NK cells.
- Maintain a physiologically relevant environment, preserving natural cell-cell interactions.
- Suitable for screening, profiling, and mechanistic studies, offering flexibility across research stages.
- Useful as a cost-effective first-line assay to prioritize compounds and guide further investigation.
PBMC assays are a valuable tool for researchers aiming to understand immune modulation in a complex cellular setting, supporting both exploratory and hypothesis-driven studies.
Isolated immune cell assays
Focused analysis for cell-type specific investigations
Monoculture assays using isolated or differentiated immune cells, such as T cells, B cells, NK cells, monocytes, dendritic cells, and macrophages, offer a valuable approach for studying the activity of compounds on specific cell types. These assays allow researchers to assess target engagement, cell-specific effects, and begin to explore the mechanism of action in a controlled setting.
- Enable detailed analysis of signaling pathway activation and gene expression changes.
- Help confirm whether observed effects are cell-type specific or more broadly distributed.
- Provide insight into off-target activity and secondary effects, supporting a more comprehensive understanding of compound behaviour.
- Useful for refining hypotheses, validating targets, and informing the direction of therapeutic development.
This assay format supports deeper mechanistic studies and can complement broader immune profiling approaches, helping researchers build a clearer picture of how a compound interacts with the immune system.
Co-culture assays
Modeling cellular interactions for mechanistic and translational insights
Co-culture assays provide a valuable platform for studying interactions between immune and non-immune cells, offering a more physiologically relevant model to investigate target biology, compound effects, and potential biomarker discovery. These assays can be performed in 2D or 3D formats, using direct or indirect contact setups, depending on the research question.
- Enable investigation of cell-cell communication and cross-talk in a controlled environment.
- Capture complex immune responses and their influence on other cell types, such as epithelial or cancer cells.
- Support mechanistic studies and help identify net effects of target modulation across multiple cell types.
- Useful for exploring system-level responses that are not apparent in monoculture assays.
Examples of established co-culture models include:
- Macrophage–T cell interactions
- Macrophage–epithelial cell systems
- PBMC–cancer cell co-cultures
- Fibroblast–T cell models
These assays require careful design and a high level of technical expertise, but they offer a more integrated view of immune function, often better reflecting in vivo-like conditions. They are particularly useful for researchers aiming to understand multicellular dynamics and refine translational strategies.
Areas of expertise
Immuno-oncology
Our team brings over 10 years of experience in developing assays that model key aspects of immune cell interactions with cancer, supporting both mechanistic studies and therapeutic development.
Modeling T cell exhaustion phenotypes
We develop advanced T cell assays that replicate functionally distinct, exhausted T cell states commonly observed in cancer patients. These models are ideal for studying the mechanisms of T cell exhaustion, identifying novel immunotherapy targets, and evaluating compound efficacy in reversing dysfunctional T cell responses.
Tumor-associated macrophage (TAM) co-culture systems
Our co-culture assays are designed to assess how compounds influence the reprogramming of TAMs and their downstream effects on T cell suppression. These models help elucidate the immunosuppressive tumor microenvironment and support the development of strategies to restore effective anti-tumor immunity.
3D Immuno-oncology models
We build complex 3D assay systems incorporating cancer cells, cancer-associated fibroblasts, and immune cells to better mimic the tumor microenvironment. These models provide a more physiologically relevant platform for testing compound activity, enabling deeper insights into therapeutic potential and translational relevance.
Autoimmunity
Our team develops specialized in vitro models to investigate immune mechanisms underlying autoimmune diseases and evaluate the therapeutic potential of novel compounds.
B Cell activation assays
We use isolated B cells from PBMCs and stimulate them with disease-relevant agents to model B cell hyperactivity seen in autoimmune conditions. These assays allow assessment of a compound’s ability to suppress B cell activation, with readouts including proliferation, activation marker expression, differentiation, and antibody production.
Dendritic cell modulation assays
These assays evaluate how compounds influence dendritic cell maturation and their capacity to activate antigen-specific T cells. Readouts include maturation marker expression and cytokine release, providing insight into a compound’s potential to dampen immune activation at the antigen presentation stage.
These models support mechanistic studies and compound screening in the context of immune tolerance, inflammation, and autoimmune pathogenesis, helping researchers better understand therapeutic mechanisms and refine development strategies.
Inflammation
We offer a range of well-characterized assays to model key inflammatory processes, enabling detailed evaluation of compound effects on immune cell function and inflammatory signalling.
T Cell differentiation assays
We model T cell polarization under defined culture conditions to generate TH1, TH2, and TH17 phenotypes, which are commonly associated with various inflammatory disorders. These assays are used to assess a compound’s ability to inhibit or modulate T cell differentiation, providing insight into potential anti-inflammatory mechanisms.
Macrophage phenotype modulation
Using a panel of functionally distinct macrophage subtypes, we evaluate how compounds influence macrophage polarization, particularly their shift away from pro-inflammatory or disease-associated phenotypes. This supports studies focused on immune resolution and tissue repair.
Inflammasome pathway assays
We offer a suite of assays using THP-1 cells and primary macrophages to assess compound activity on the inflammasome pathway. Readouts include ASC speck formation, IL-1β release, and other markers of inflammasome activation, enabling detailed profiling of anti-inflammatory potential.
Support from concept to clinic
Our specialist immunology translational biology expertise is complemented by a wider offering across chemistry, ADMET, and GMP manufacturing services. Speak to our team to explore how we can help you bring your immunology therapeutic to the clinic.





