Neuroscience Models
Advancing drug development for neurodegenerative diseases
.jpg)
Unlocking integrated cell-based assays for drug discovery in neuroscience
Innovative translational models are essential to accurately predict central nervous system (CNS) therapeutic outcomes, providing a robust dataset to derisk drug development. Our team of expert neuroscientists bring decades of experience leveraging human iPSC-derived systems, rodent primary CNS cells and organotypic models to accelerate your journey from target to optimized lead.
Our model systems
A challenge facing neurodegenerative disease therapy development is identifying appropriate and robust experimental models in which to test drug efficacy, potency and toxicity. Primary human CNS cells are difficult to source and culture at scale. To overcome this challenge, we offer a suite of assays utilizing human iPSC-derived CNS cells, primary rodent CNS cells, CNS cell lines and organotypic brain slice cultures.
We offer consultative insight to drive your program forward, including comprehensive high-dimensional readouts including:
- Live cell imaging
- Immunofluorescence
- High Content Imaging
- Quantitative analysis of gene and protein expression
- Targeted metabolomics
We integrate across all service lines, including immunology, spatial biology, molecular biology, biophysics, biomarker analysis and ADMET & DMPK to ensure that you meet legislative requirements for you candidate drugs.
Human iPSC derived CNS cells
Human iPSC-derived CNS cells provide an excellent alternative to primary human CNS cells, which are difficult to source and culture at scale. Their gene expression profile and functional phenotypes closely align with that of their in vivo counterparts, making them the most physiologically representative model of human CNS cells that currently exists.
Gene editing technologies can be applied to generate lines carrying disease-associated mutations and isogenic control lines, making them an ideal tool for translational CNS disease modeling and drug discovery.
We are experts at harnessing the power of iPSC-derived neurons, astrocytes and microglia and we can design assays tailored to your individual requirements.
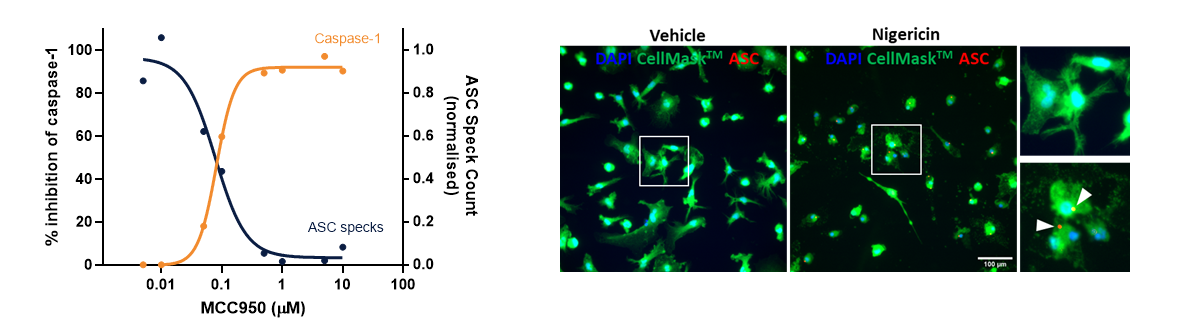
Primary CNS cells
Understanding the effects of candidate drugs on primary cells is a key step in your CNS drug discovery program.
Unlike primary human CNS cells, primary rodent CNS cells can be sourced and cultured with relative ease. They are a biologically representative of in vivo cell physiology, and as such they are a valuable experimental platform to support the transition to in vivo studies, especially in rodent models.
Primary cells can be sourced from wild-type animals, as well as genetically modified lines, and cultured in the presence of candidate therapeutics. We have extensive experience of isolating and culturing primary rodent CNS cells to help you understand the interaction between your drug and the nervous system.
We purify and culture rodent:
- Microglia
- Astrocytes
- Neurons
- Oligodendrocyte Precursor cells (OPC)
CNS cell lines
Immortalized CNS cell lines can be a valuable and cost-effective model system in which to conduct primary screens of compound libraries.
We have extensive experience of harnessing the versatility of cell lines for drug discovery applications and can design and execute assays tailored to your target and therapeutic modality.
Organotypic brain slice culture
Brain slice cultures contain and maintain all the relevant neuronal and glial cells, structure, and organization of the brain in thick sections of cultured ex vivo tissue. This versatile 3D model offers a powerful translational link between in vitro and in vivo CNS studies in a variety of indications. Exemplary applications of organotypic brain slices include:
- Neuroinflammatory activation
- Inflammasome activation
- Neurotoxicity
- Neuroprotection
- Developmental myelination
This biological system is ideally suited for a low-throughput validation of your test compound in a complex multicellular CNS model.