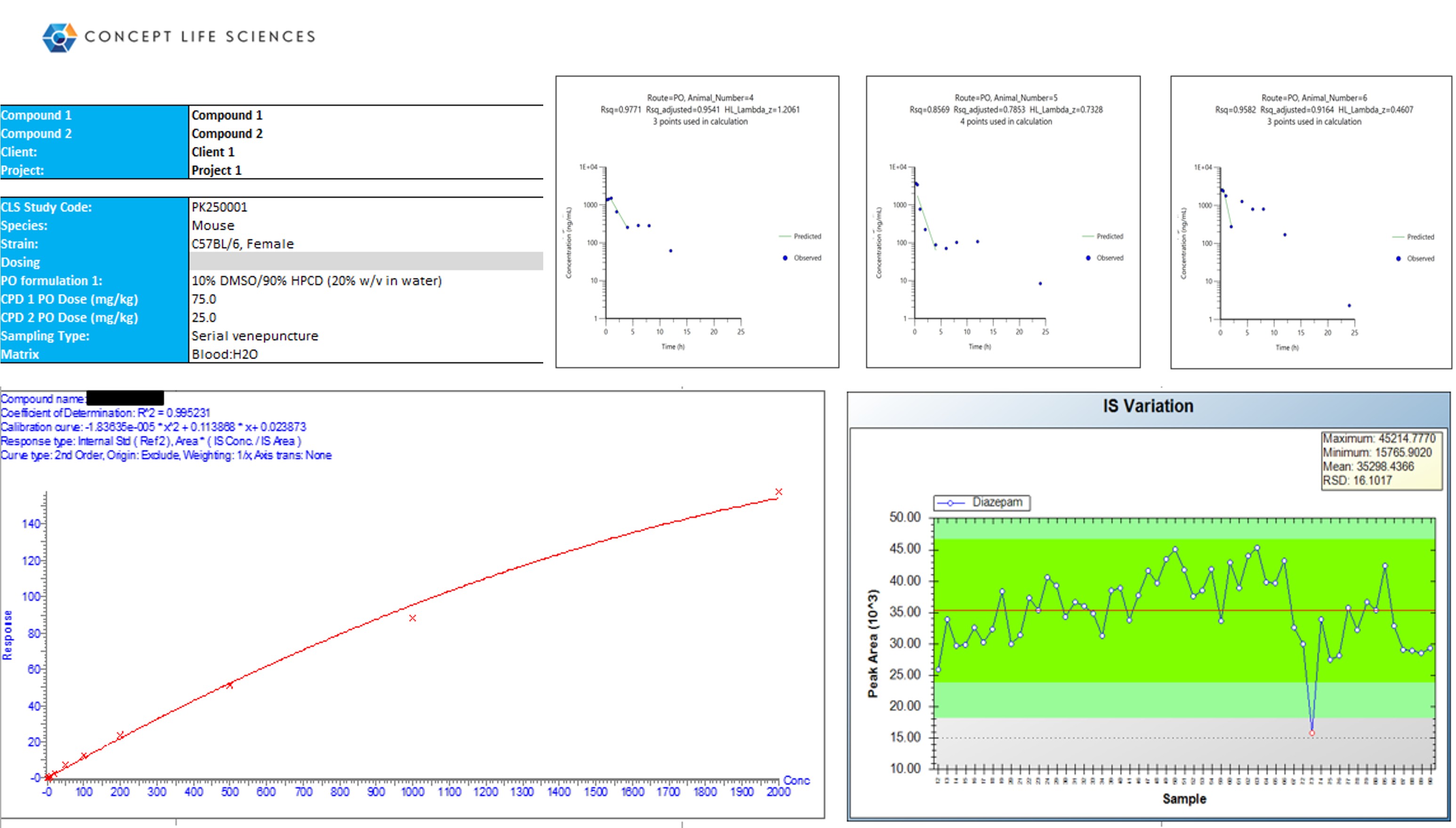
In Vivo Pharmacokinetics (PK) Screen
Rapid, cost-effective early-stage DMPK testing

How do I accelerate early-stage DMPK decision-making?
Early in vivo pharmacokinetics (PK) screening provides fast, affordable insight into your compound’s behavior, helping you make confident drug metabolism and pharmacokinetics (DMPK) decisions before investing in complex in vivo studies. By working with us you can customize your service and avoid costly delays in your drug development program.
Why early PK screening is critical for drug development
When working to advance a molecule from concept to clinic, you need PK and bioanalysis support that adapts to your project’s complexity, timelines and budget.
Our customized services focus on clarity, speed and quality, so you can:
- Identify analytical challenges such as matrix interference, ion suppression or binding issues.
- Make efficient data-driven decisions confidently.
- Optimize compound selection and streamline project timelines.
In vivo PK screening workflow: Speed, clarity and quality
Concept Life Sciences offers cost-effective, customized PK screening services that identify analytical challenges long before expensive in vivo studies begin. Using minimal material and focusing on compatibility and optimisation you can avoid costly delays in your drug development program.
Limited material, high risk.
Determine compound tractable.
Uses ≤1 mg: you supply the compound; we handle the rest.
Unexpected analytical issues.
Detect ion suppression or binding artifacts early.
LC/MS optimization under generic conditions.
Unclear method development needs.
Understand the scale of future analytical work.
Quantitative assessment of method development requirements.
High study costs and ethical concerns.
Avoid unnecessary animal dosing.
Early compatibility insights prevent wasted in vivo work.
Tight timelines.
Rapid, actionable results.
Preliminary data in one day; full PK report in 3–5 days.
Learn more about our bioanalytical service tiers
Real-world success: PK Screen case study
Client: UK Biotech DMPK Group
Challenge: Screening a compound across two matrices, with the intention to expand to full sample analyses if the LLOQ criteria could be satisfied. They had repeatedly failed internal assays due to interference from structurally similar compounds.
Solution: Our screening identified an unsuitable matrix due to endogenous interference but confirmed LLOQ in an alternative matrix.
Impact: Early insights prevented wasted effort and guided the client to proceed confidently with full analysis under our support.
Why Concept Life Sciences for PK screening?
A streamlined PK screening workflow sits alongside our tiered bioanalytical service structure, so you can choose the right level of support to match your needs, budget and timeline for each stage of your project.
Proven DMPK expertise: Decades of experience developing and customizing chromatographic methods for small molecules.
Flexible service structure: Tiered bioanalytical workflows that match your timeline, budget, and regulatory needs.
Integrated continuity: Support from early discovery through GLP and GCP phases ensures consistency and data integrity.
Collaborative partnership: Direct access to scientists who provide clear guidance and actionable insights.
Cost-effective decision-making: Minimize compound use while accelerating discovery.
Start your PK strategy today
In vivo PK screening FAQs
Q: What is PK screening?
A: Pharmacokinetics (PK) screening is an early-stage compatibility test that evaluates how your compound behaves in biological matrices, helping you anticipate analytical challenges before full in vivo dosing.
Q: How much compound do I need?
A: Only 1 mg (or less) of compound is required for a complete PK screen.
Q: How quickly will I receive results?
A: Preliminary results are available within one working day and a comprehensive analytical report follows in 3–5 days.
Q: Why perform PK screening before in vivo studies?
A: Early screening prevents costly late-stage failures by identifying ion suppression, matrix interference and binding issues that could compromise in vivo data quality.
