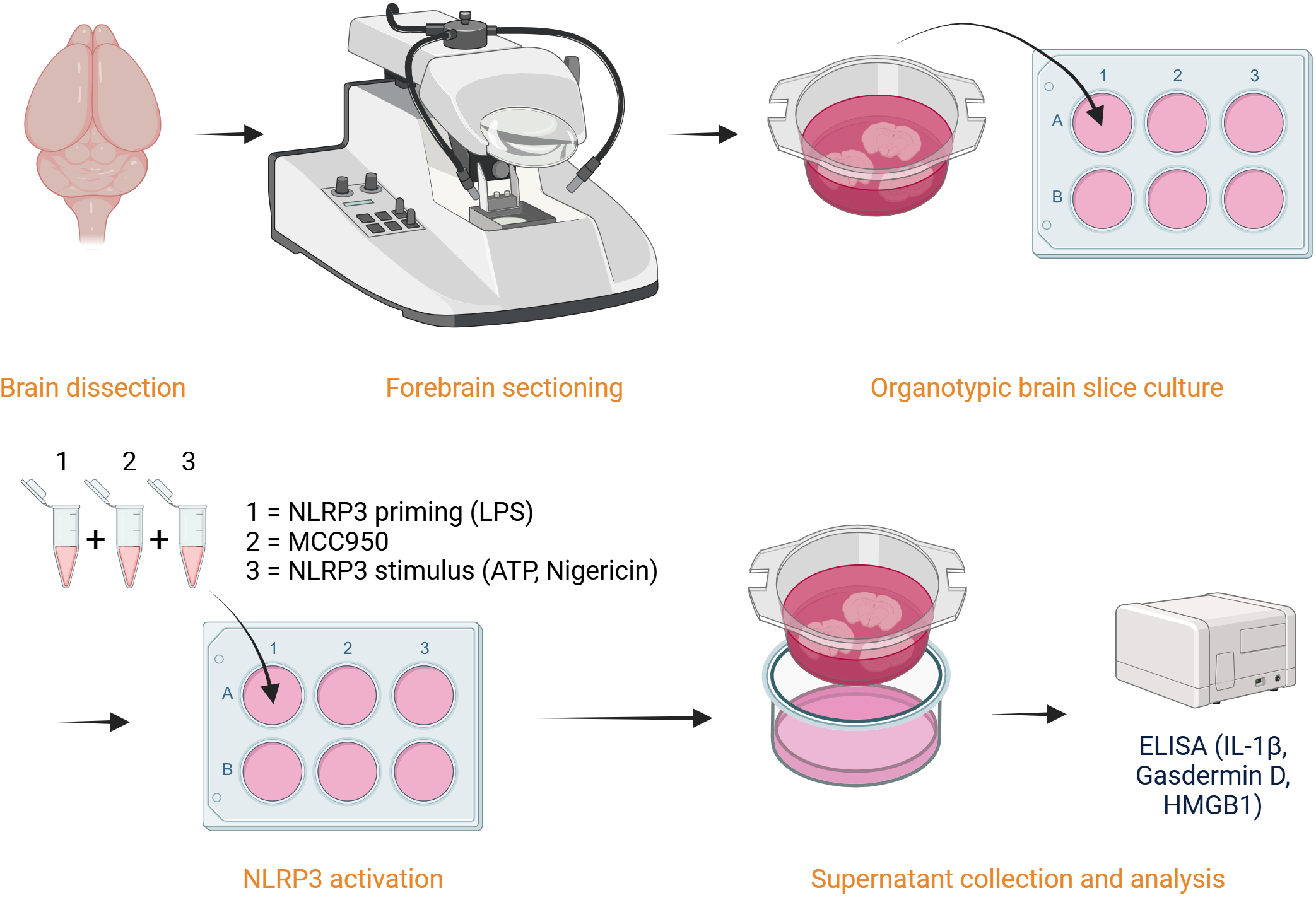
Organotypic Brain Slice Inflammasome Assay

Test the efficacy of your novel NLRP3 inhibitors in a physiologically relevant ex vivo system
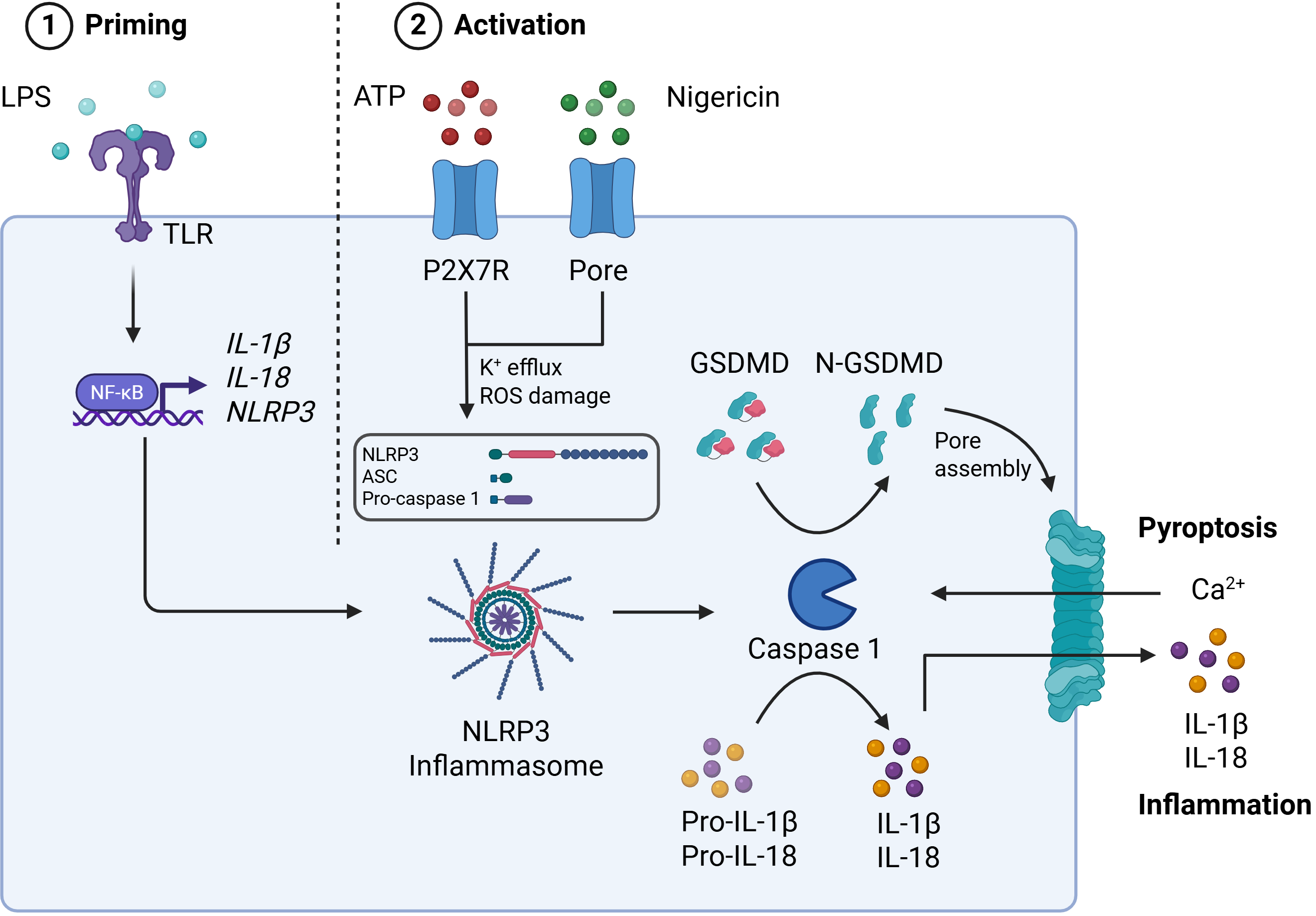
Inflammasomes are large intracellular protein complexes that assemble in response to danger signals and function as key components of the innate immune system. Once activated, they drive Caspase-1–mediated secretion of IL-1β and IL-18 and induce pyroptosis via cleavage of Gasdermin D (Figure 1).
Among inflammasomes, NLRP3 is a well-validated therapeutic target for neurodegenerative disease. It responds to a wide range of damage- and pathogen-associated stimuli, and its chronic activation is implicated in CNS inflammation and pathology.
Key benefits
Our brain slice inflammasome assay provides a powerful 3D ex vivo platform to assess efficacy of NLRP3 inhibitors in a native CNS environment.
- Preserves key CNS cell populations and cytoarchitecture for physiologically relevant insights
- Enables robust, reproducible readouts of inflammasome activation and inhibition
- Supports a range of end-point analyses including ELISA, western blot, immunohistochemistry, qPCR, and RNAseq
- Cost-effective and faster than in vivo models
- Adaptable to other inflammasome pathways and disease mechanisms
Protocol
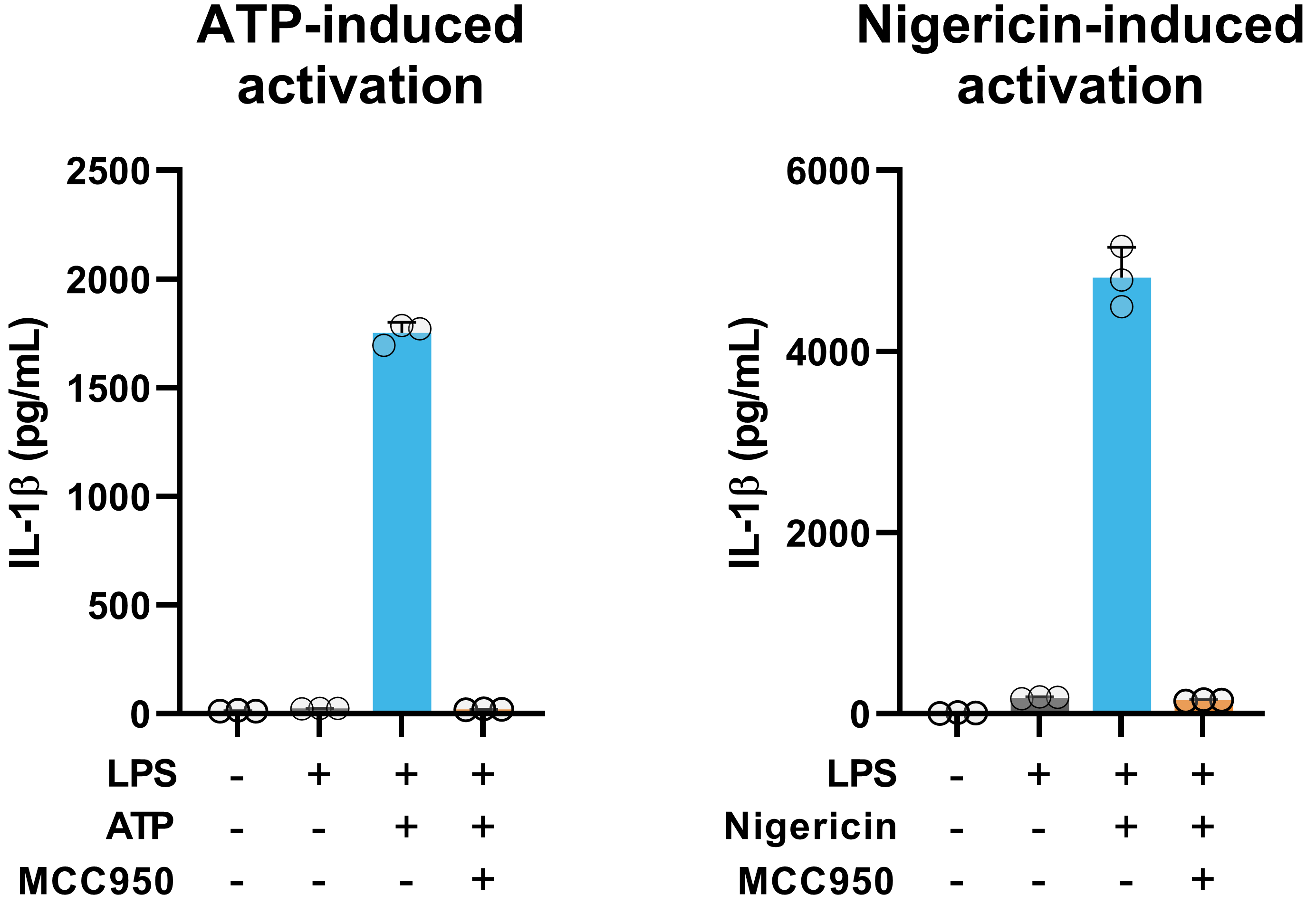
Data analysis and results
Selective activation of the NLRP3 inflammasome with ATP or Nigericin induces robust IL-1β secretion
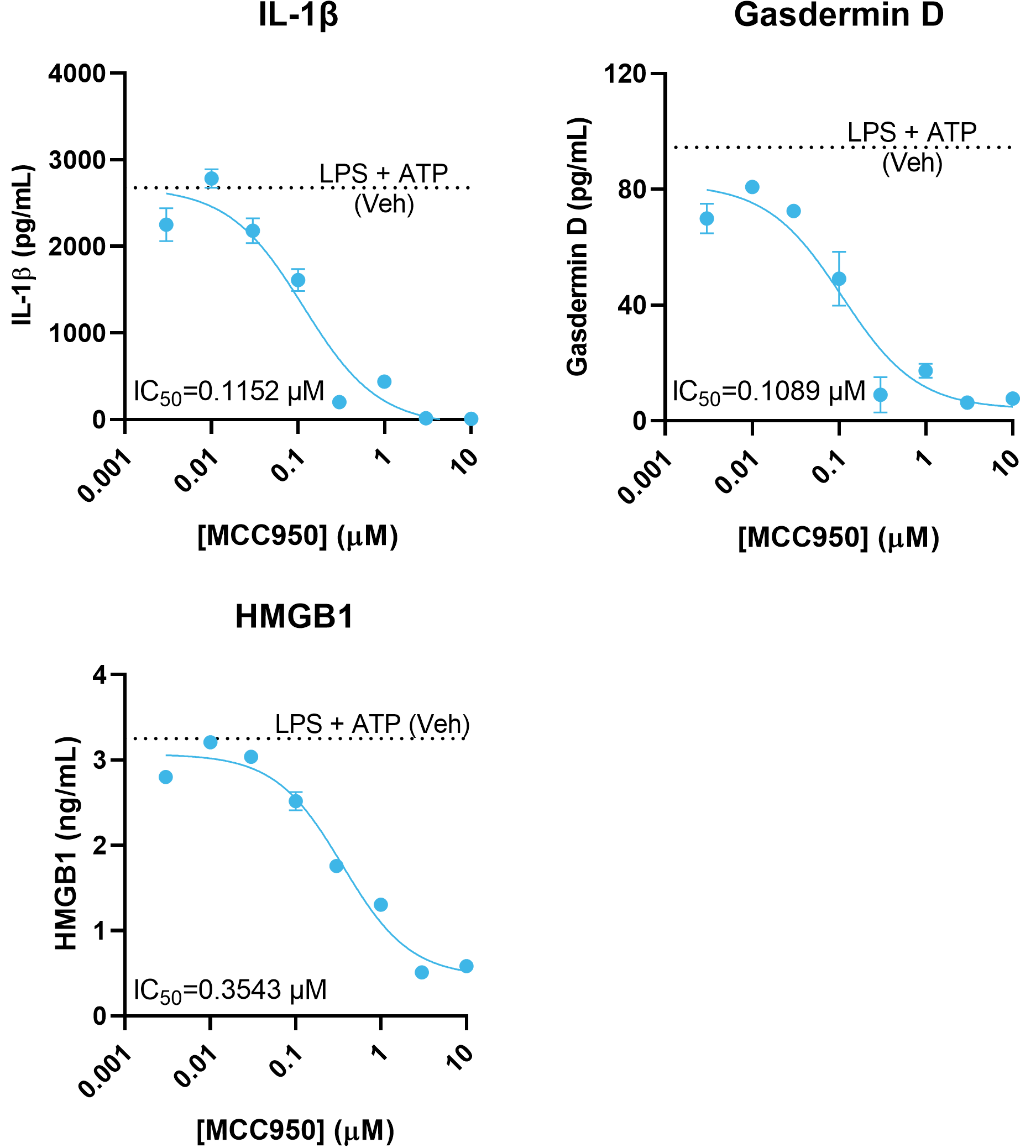
The secretion of multiple NLRP3 activation markers is concentration-dependently inhibited by MCC950
After supernatant collection, brain slices can be utilized for various end-point readouts, including western blotting, immunohistochemistry, RTqPCR, and RNAseq
Conclusions
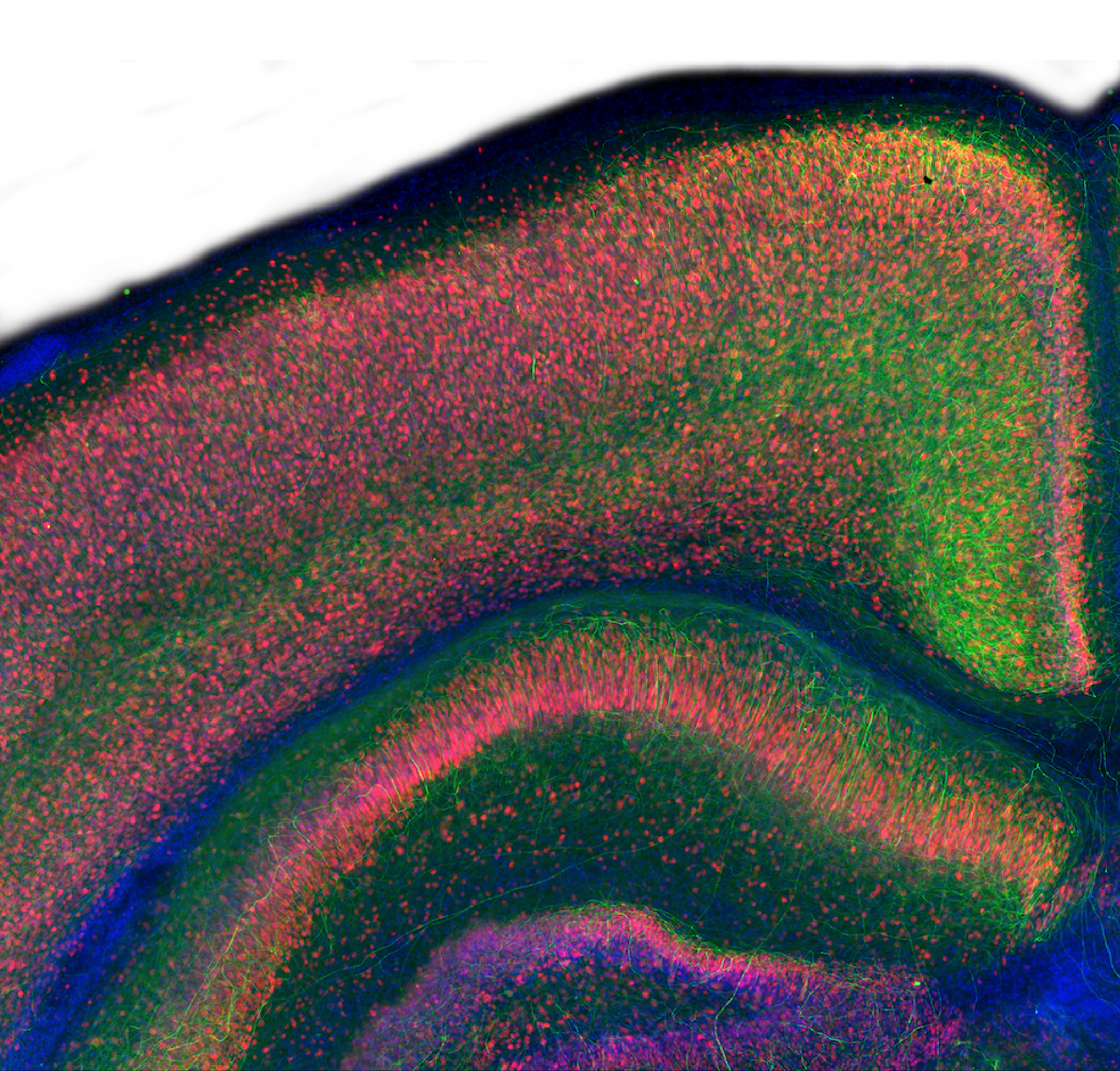
NLRP3 activation contributes to the neuroinflammation that drives neurodegenerative diseases such as Alzheimer’s and Parkinson’s. Our organotypic brain slice assay captures key aspects of CNS inflammasome biology, enabling accurate assessment of NLRP3 inhibitors in a complex and native tissue environment.
By offering this physiologically relevant platform, we support in-depth compound profiling and mechanism-of-action studies; de-risking your in vivo studies and helping you progress promising candidates with confidence.
