Expert Knowledge
Mechanistic Toxicology for Endocrine and Thyroid Disruption
Navigating regulatory expectations with confidence
Endocrine (ED) and thyroid disruption (TD) have moved from a niche scientific curiosity to a central pillar of chemical safety assessment. Across global regulatory frameworks, the expectation has shifted: it is no longer sufficient to observe an effect; companies must explain it biologically and mechanistically (Bergman et al., 2013; EFSA, 2018). Early mechanistic insight ensures that observed biological signals are contextualized, relevant and defensible thus supporting hazard classification, risk assessment and regulatory submissions (Doerr et al., 2024).
Regulatory stakes are high:
- Early ED conclusions can propagate across frameworks, influencing product classification, mixture labeling, and risk management.
- Default assumptions on human-relevance and thyroid perturbations place the burden of proof on registrants.
- Uncertainty can lead to conservative regulatory decisions, delayed approvals and/or additional testing requirements.
Mechanistic toxicology bridges this gap between observed effects and regulatory interpretation and providing clarity for hazard classification, risk assessment and submission confidence.
The current regulatory reality: Why mechanistic evidence is now expected
Endocrine and thyroid disruption are no longer peripheral. Across global frameworks, expectations have shifted decisively, from identifying effects to explaining them (EFSA, 2018; OECD, 2018). Internationally, endocrine disruption is recognized as a global concern, with harmonized guidance, tiered testing strategies, and coordinated monitoring initiatives supporting the early identification and prioritization of potentially hazardous substances (OECD, 2018; Kassotis et al., 2020). Globally, approaches vary:
- Risk-based evaluations weigh potency, exposure, and human relevance, applied case-by-case.
- Hazard-based approaches particularly in Europe, assume human relevance unless mechanistic data convincingly disproves it. Thyroid endpoints are treated as human-relevant by default.
Recent developments instigated within the EU regulatory framework reinforce this trend:
CLP centralization: One-substance, one-assessment (OSOA) logic ensures ED conclusions propagate across sectors, limiting reinterpretation (CLP, 2008; ECHA, 2018; ECHA 2024).
PPP and REACH alignment efforts: Mechanistic clarity is critical to anticipate classification, renewal outcomes, and regulatory prioritization (EC 1107/2009; EFSA, 2018).
REACH prioritization and upcoming updates: With ~23,000 registered substances, mechanistic data inform screening and substance evaluation, preventing unnecessary regulatory escalation. ECHA updates are expected soon in 2026 affecting how endocrine disruption is assessed and which substances may require additional data. Companies should anticipate these updates and prepare mechanistic dossiers accordingly.
Key regulatory messages embedded in current guidance:
- Evidence of activity + adversity + biologically plausible link is required for classification (OECD, 2018). Current guidance, including OECD Guidance Document 150 (2018), supports the integration of mechanistic data, in vitro assays, and adverse outcome pathways (AOPs) to establish biologically plausible links for regulatory decision-making.
- Mechanistic data, in vitro assays, and adverse outcome pathways (AOPs) are accepted and encouraged to establish human or environmental relevance (OECD, 2018; EFSA, 2018).
- Weight-of-Evidence (WOE) approaches remain central, but favor regulatory caution is favoured by default in cases of uncertainty.
Mechanistic uncertainty carries real consequences: substances may face classification, delayed renewal and/or additional testing if mechanistic clarity is missing. For regulatory teams, mechanistic toxicology is no longer optional; it is a strategic tool to reduce risk and guide decision-making.
Mechanistic insight: understanding endocrine and thyroid biology
The endocrine and thyroid systems are finely tuned networks of receptors, enzymes, transporters, and feedback loops. Disruption can occur at multiple points, each representing a molecular initiating event (MIE) that experimental assays can target (Bergman et al., 2012; Martiyniuk et al., 2022). Figure-1 provides an integrated overview of endocrine (ER/AR, steroidogenesis) and thyroid pathways (collectively labelled as EATS), showing where chemicals can interfere and the mechanistic rationale for observed outcomes:
- EAS pathways (estrogen/androgen/steroidogenesis):
Ligand binding - Receptor activation - Gene transcription - Downstream reproductive effects.
Full EATS coverage captures the complete mechanistic spectrum (OECD TG 456, 457, 458; Holbech et al., 2020). Perturbation of steroidogenesis occurs through inhibition of key enzymes (CYP17, CYP19/aromatase, 3β-HSD), affecting testosterone, estradiol, or progesterone synthesis. These pathways integrate with hypothalamic-pituitary-gonadal (HPG) feedback loops, so even a subtle modulation can amplify downstream effects. Mechanistic assays detect these MIEs, providing biologically plausible explanations for observed reproductive or developmental effects.
- Thyroid pathways:
Thyroid hormones (THs) are essential regulators of metabolism, neurodevelopment and growth. Maintaining their homeostasis is critical for health across all life stages. Disruption of TH signaling, especially during development, can lead to irreversible neurocognitive deficits and metabolic disorders. Environmental chemicals and xenobiotics can interfere with TH biosynthesis, metabolism or clearance, acting at distinct molecular initiating events (MIEs). Traditional in vivo rodent assays have limitations due to interspecies differences in TH physiology (e.g. hormone transport, metabolism, feedback loops). These models also overlook extra-thyroidal mechanisms including hepatic metabolism and peripheral hormone conversion. There is a growing need for human-relevant, mechanism-based in vitro assays aligned with New Approach Methodologies (NAMs) to improve regulatory assessment and reduce animal use. TH-related pathways include hormone synthesis (TPO), transport (TTR), uptake (NIS), metabolism (UGT/DIO) and feedback regulation. Thyroid Molecular Initiating Events (MIEs) provide a mechanistic basis for interpreting modest hormone changes in toxicology studies (Noyes et al. 2019) to allow interpretation of how chemicals alter thyroid homeostasis at multiple levels. TPO inhibition limits T4/T3 synthesis, DIO inhibition prevents peripheral conversion of T4 to T3, NIS inhibition blocks iodide uptake, and alterations in TTR or UGT affect hormone transport and metabolism. Feedback via the hypothalamic-pituitary-thyroid (HPT) axis may compensate, resulting in subtle serum changes, but tissue-level effects may still be significant. Mechanistic assays, therefore, contextualize observed hormonal changes within a biologically plausible framework.
For example, a compound may inhibit TPO without changing circulating thyroid hormones or bind weakly to ER without transcriptional activation. Mechanistic assays therefore provide critical insight for regulatory interpretation.

How mechanistic assays address regulatory questions
Mechanistic assays translate biological insight into regulatory evidence, supporting weight-of-evidence conclusions and risk management. They are critical for both in vitro and integrated in vivo / ex vivo strategies (Table 1).
- ER/AR binding and transactivation detect receptor-mediated effects.
- Steroidogenesis and aromatase inhibition highlight upstream hormone perturbation.
- Thyroid MIE assays elucidate potential points of thyroid disruption.
- Ex vivo and in vivo assays validate these mechanisms at tissue and systemic levels, confirming feedback loop interactions and species relevance.
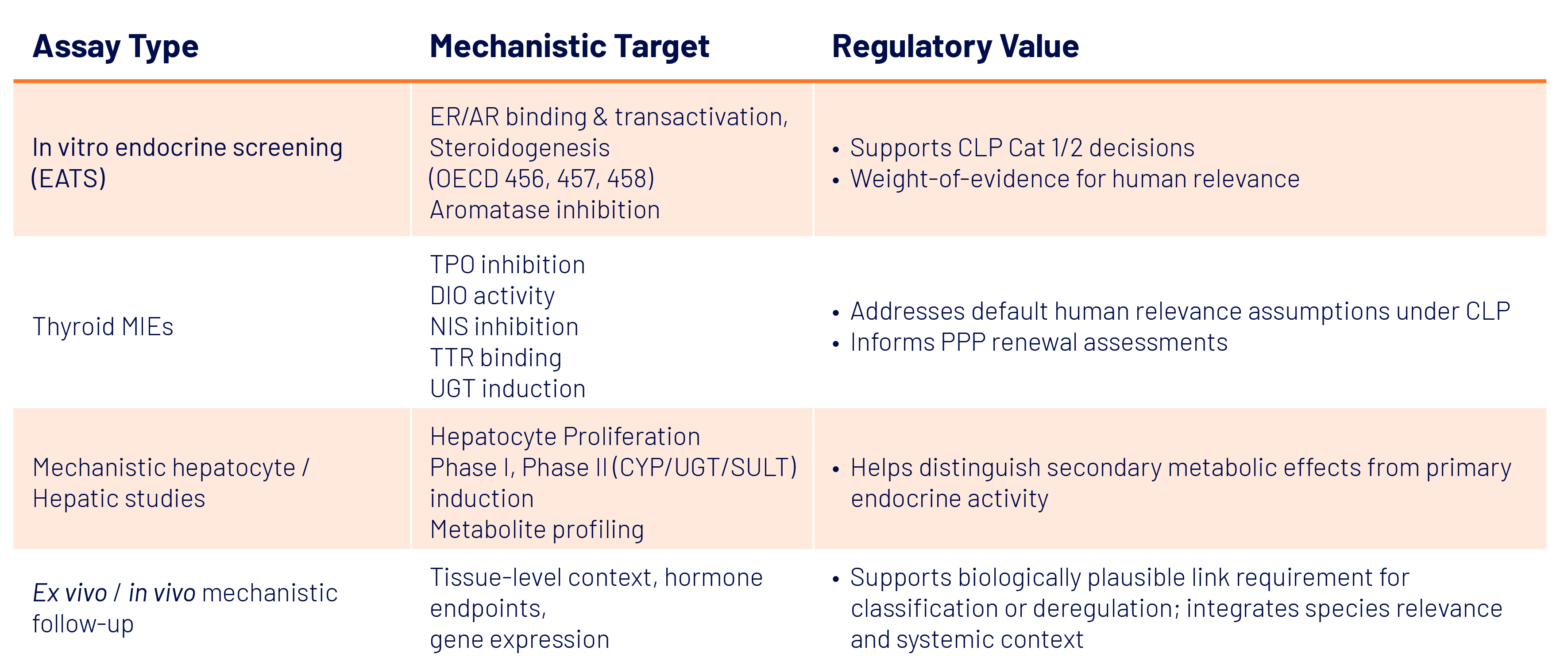
These assays provide defensible mechanistic inputs that regulators expect, enabling more targeted testing, reducing animal use, and strengthening submission robustness.

Practical applications for R&D and regulatory teams
Mechanistic toxicology supports decision-making across the chemical lifecycle:
Early screening: Identify liabilities, guide structural modifications, refine candidate selection.
Regulatory submissions: Supports endocrine disruptor evaluations, thyroid mode-of-action interpretation, REACH Annex requirements, and EFSA pesticide assessments.
Explaining unexpected findings: Distinguish primary endocrine disruption from secondary metabolic effects, particularly liver-mediated effects.
Reducing regulatory risk: Mechanistic clarity prevents misclassification, unnecessary testing, and submission delays.
What Concept Life Sciences can do to help
Our scientific team helps clients design mechanistic testing strategies aligned with global regulatory expectations. We offer integrated EATS and thyroid assays, in vivo and ex vivo studies, and weight-of-evidence support, producing defensible, regulator-ready data. These top-level solutions reduce ambiguity, anticipate regulatory queries, and guide strategic decision-making.
For more details on services, visit our Endocrine and Thyroid Testing page.
References:
- Bergman et al. The impact of endocrine disruption: a consensus statement on the state of the science. Environ Health Perspect. 2013;121(4).
- EFSA. Guidance for the identification of endocrine disruptors in the context of Regulations (EU) No 528/2012 and (EC) No 1107/2009. EFSA J. 2018.
- Doerr et al. A UK framework for the assessment and integration of different scientific evidence streams in chemical risk assessment. Regul Toxicol Pharmacol. 2024; 151:105652.
- OECD. Revised Guidance Document 150 on Standardised Test Guidelines for Evaluating Chemicals for Endocrine Disruption. OECD Series on Testing and Assessment, No. 150. Paris: OECD Publishing; 2018.
- Kassotis et al. Endocrine-disrupting chemicals: economic, regulatory, and policy implications. Lancet Diabetes Endocrinol. 2020;8(8):719–730.
- EC. Regulation (EC) No 1272/2008 on classification, labelling and packaging of substances and mixtures. OJ L 353. 2008:1–1355.
- ECHA. Guidance for the identification of endocrine disruptors in the context of Regulations (EU) No 528/2012 and (EC) No 1107/2009. EFSA J. 2018.
- ECHA. Guidance on the Application of the CLP Criteria: Endocrine Disruptors, first update, November 2024.
- EC. Regulation (EC) No 1107/2009 concerning the placing of plant protection products on the market. OJ L 309. 2009:1–50.
- EU. Regulation (EU) No 528/2012 concerning the making available on the market and use of biocidal products. OJ L 167. 2012:1–123.
- Martyniuk et al. Emerging concepts and opportunities for endocrine disruptor screening of the non-EATS modalities. Environ Res. 2022; 204:111904.
- Holbech et al. ERGO: Breaking down the wall between human health and environmental testing of endocrine disrupters. Int J Mol Sci. 2020; 21:2954.
- Noyes et al. Evaluating chemicals for thyroid disruption: opportunities and challenges with in vitro testing and adverse outcome pathway approaches. Environ Health Perspect. 2019; 127:95001. doi:10.1289/EHP5297
Abbreviations:
AR: Androgen Receptor; CLP: Classification, Labeling and Packaging Regulation; CYP: Cytochrome P450; DIO: Deiodinase; ER: Estrogen Receptor; EAS: Estrogen-Androgen-Steroidogenesis; EATS: Estrogenic-Androgenic-Thyroid-Steroidogenic; ECHA: European Chemicals Agency; EFSA: European Food Safety Authority; HPT: Hypothalamic-Pituitary-Thyroid axis; MIE: Molecular Initiating Event; NAM: New Approach Methodologies; NIS: Sodium Iodide Symporter; OECD: Organization for Economic Cooperation and Development; REACH: Registration, Evaluation, Authorisation and Restriction of Chemicals; SULT: Sulfotransferases; TG: Test Guideline; TH: Thyroid Hormone; TPO: Thyroid peroxidase; TTR: Transthyretin; UGT: Uridine 5'-diphospho-glucuronosyltransferase; WOE: Weight Of Evidence.

















