Production of a G-protein Coupled Receptor (GCPR) Protein to Assess Target engagement
Successful expression and purification of a functional GPCR for a downstream biophysical assay

Project overview
The challenge
- No commercial availability of membrane spanning TGR5 domain.
- Expression in bacterial cells would result in non-functional protein.
- Production of high yield, pure, active membrane proteins and measurement of target engagement is notoriously challenging.
Our solution
- Design and engineering of a tagged, fusion construct suitable for downstream biophysical assays.
- Delivery of over 75% pure and functional protein, able to bind a positive control lipidic ligand.
- Use of a cysteine-reactive dye for thermal shift analysis.
Our impact
- Delivered fit-for-purpose protein which was unavailable commercially purchased.
- Protein activity proven through functional stabilization using a positive control ligand.
- Thermal shift measured target engagement with only microgram protein requirement.
“We came up against a challenge with no commercial availability of the GPCR TGR5 that was suitable for ligand binding analysis. Concept’s protein scientists stepped in and led the way for us with successful production of functional protein. Thinking outside of the box, they then designed a cysteine-reactive dye TSA method for biophysical readout, enabling our team to assess target engagement. A creative team brought us answers on this challenging target!"
Toralgen Inc.
The challenge
The client wanted to assess binding of test molecules to a membrane-spanning GPCR. A suitable construct of this target protein was not commercially available with limited literature precedence. Production of membrane proteins for biophysical assays is a challenge due to low yields, low stability and the requirement for stable and functional extraction from the cell membrane.
Our approach
Recruited our membrane protein expertise – Our team of membrane protein experts reviewed the target from a structural perspective, designed an optimal construct along with associated expression and purification methodologies.
Holistic protein design – Informed construct design to ensure the protein was active and fit for all downstream purposes.
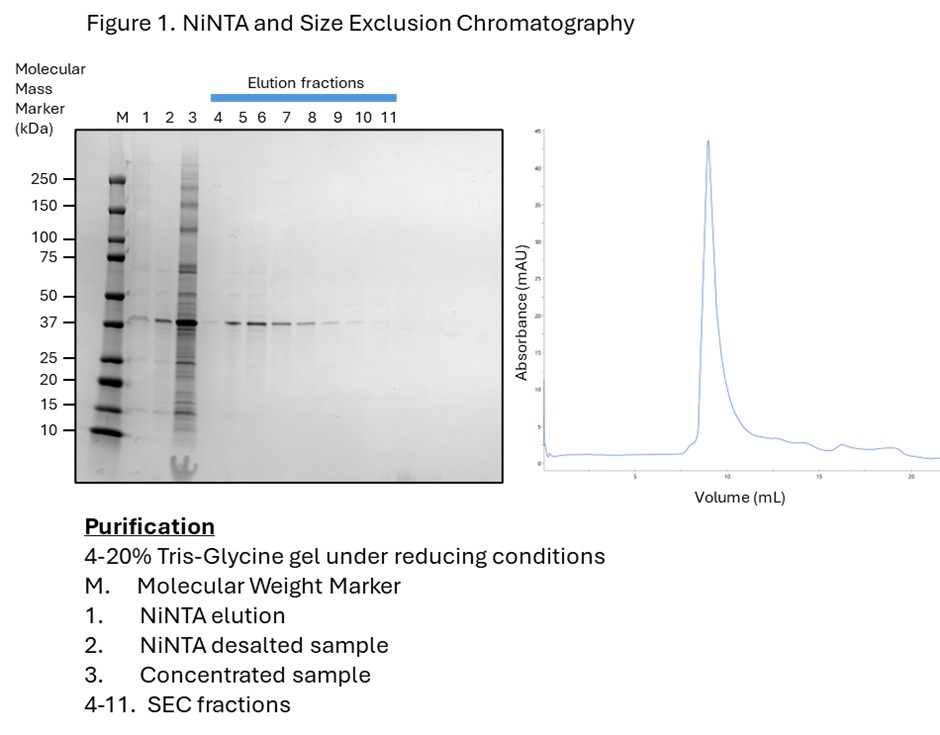
Tailored purification strategy – Use of detergent and additives using two-step purification to ensure high purity.
Bespoke activity assay – design of a dye-based thermal shift assay suitable for challenging membrane proteins.
Our solution
Using our team’s extensive experience in membrane protein production, we designed a construct with a soluble fusion domain, a C-terminal truncation and two affinity tags (FLAG and deca-Histidine) tag to aid purification. The protein was then expressed in insect cells using baculovirus transduction.
We developed a bespoke purification strategy with solubilization using LMNG detergent in the presence of the cholesterol analogue (CHS), followed by downstream purification using both anti-FLAG and NiNTA.
Using Differential Scanning Fluorimetry (DSF) we showed that the purified protein was functional. Typical DSF assays use a dye that becomes fluorescent when interacting with hydrophobic amino acids that are exposed upon the unfolding of a soluble protein. Since this was a hydrophobic membrane protein, an alternative strategy was adopted. Using our expertise coupled with the literature, we selected a dye which shows an increase in fluorescence signal upon reaction with with cysteines that are only exposed once the membrane protein unfolds. These data showed an increase in thermal stability (via an increase in melting temperature) in the presence of a know ligand, indicating that the protein was functional.
- Detergent solubilization and purification using Ni2+ affinity and Size Exclusion Chromatography resulted in pure, monodispersed protein (Figure 1).
- This purified GPCR was active, as assessed by thermal shift analysis using a known lipidic ligand (Figure 2).
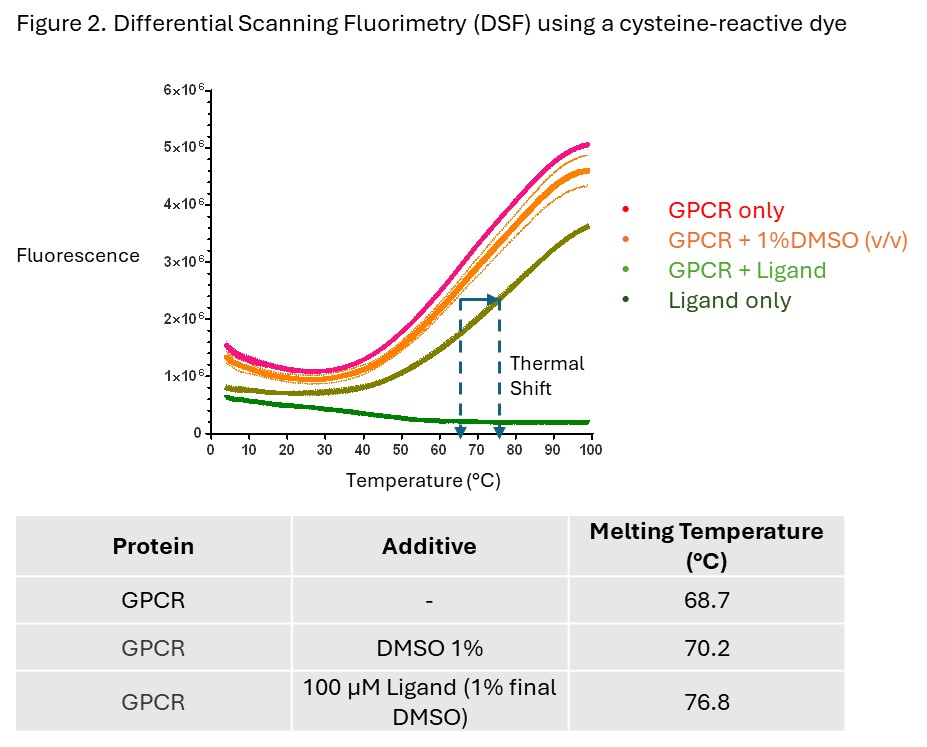
Ligand binding showed that the protein was pure, functional and suitable for downstream assays.
The result
We delivered high-quality, functional protein:
- High purity, monodispersed GPCR protein production from insect cells.
- Protein assessed as functional.
- Enabled the assessment of tool compounds using biophysical methods.
