Astrocyte Polarization Assay

Modelling neuroinflammation provides a powerful tool to test candidate drugs for anti-inflammatory and neuroprotective activity
Astrocytes are glial cells that play critical roles in maintaining central nervous system (CNS) homeostasis. Their functions include preserving the blood–brain barrier, supplying nutrients to neurons, regulating extracellular ion balance, and modulating neurotransmission.
In response to CNS injury, infection, or disease, activated microglia release pro-inflammatory cytokines that trigger astrocyte reactivity. These reactive astrocytes lose neuroprotective properties and gain neurotoxic functions, by releasing saturated lipids (Figure 1).
Neurotoxic astrocytes contribute to chronic neuroinflammation—a hallmark of many neurodegenerative diseases—and can exacerbate neuronal damage and disease progression. Modeling this process in vitro provides a powerful tool to test candidate drugs for anti-inflammatory and neuroprotective activity.

Figure 1. Pathological stimuli induce microglial activation and the release of cytokines such as TNFα, IL-1α and C1q. These factors, in turn, polarize astrocytes towards a reactive neurotoxic phenotype, which potentiates neuronal degeneration, further exacerbating neuroinflammation and disease progression.
Key benefits
- Model neuroinflammation across multiple neurodegenerative diseases
- Robust and reproducible induction of neurotoxic, pro-inflammatory astrocyte phenotypes
- Compatible with rodent and human cells; customizable by species or pathway
- Integrates with co-culture systems for multi-cellular modeling (e.g. microglia, neurons)
Protocol
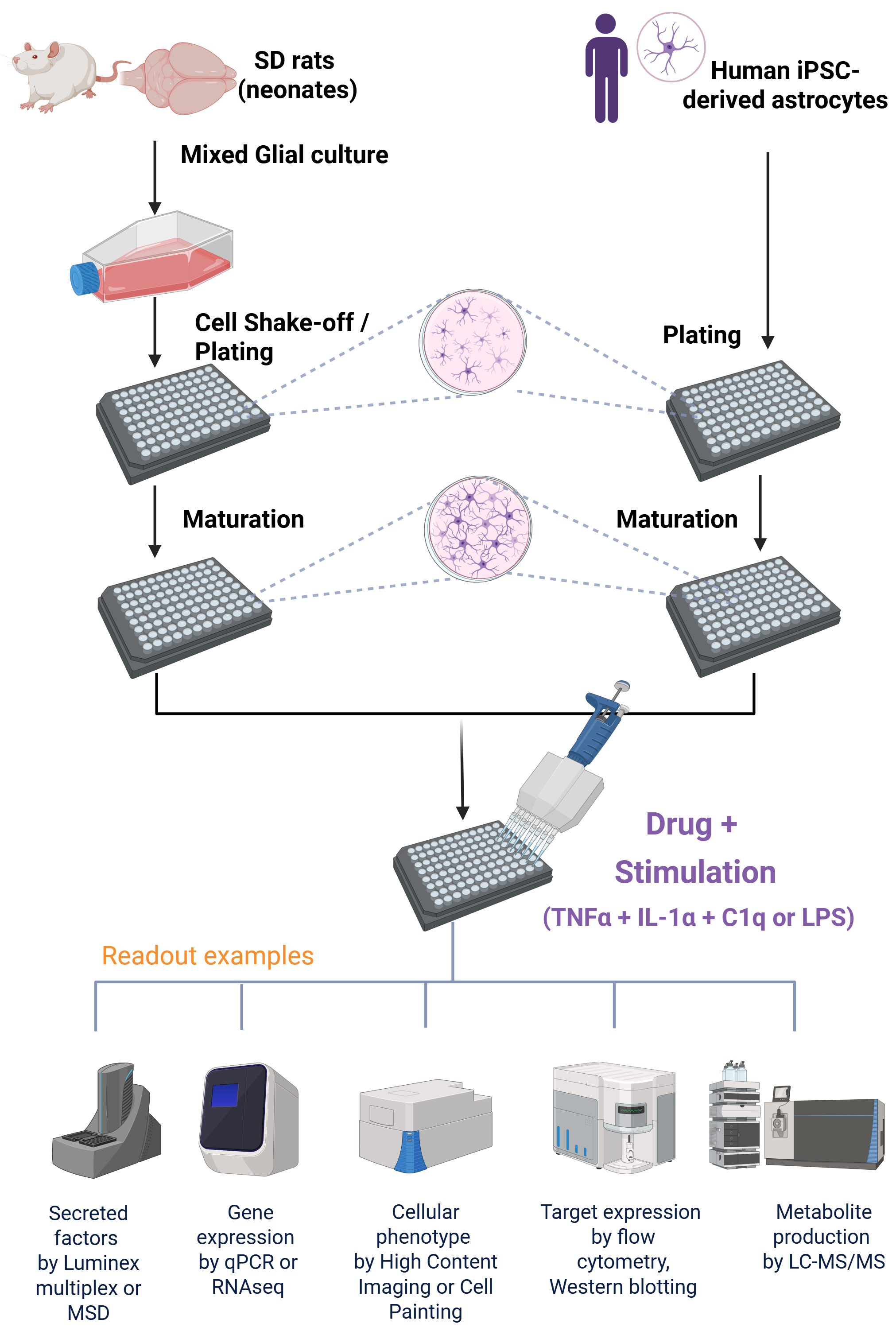
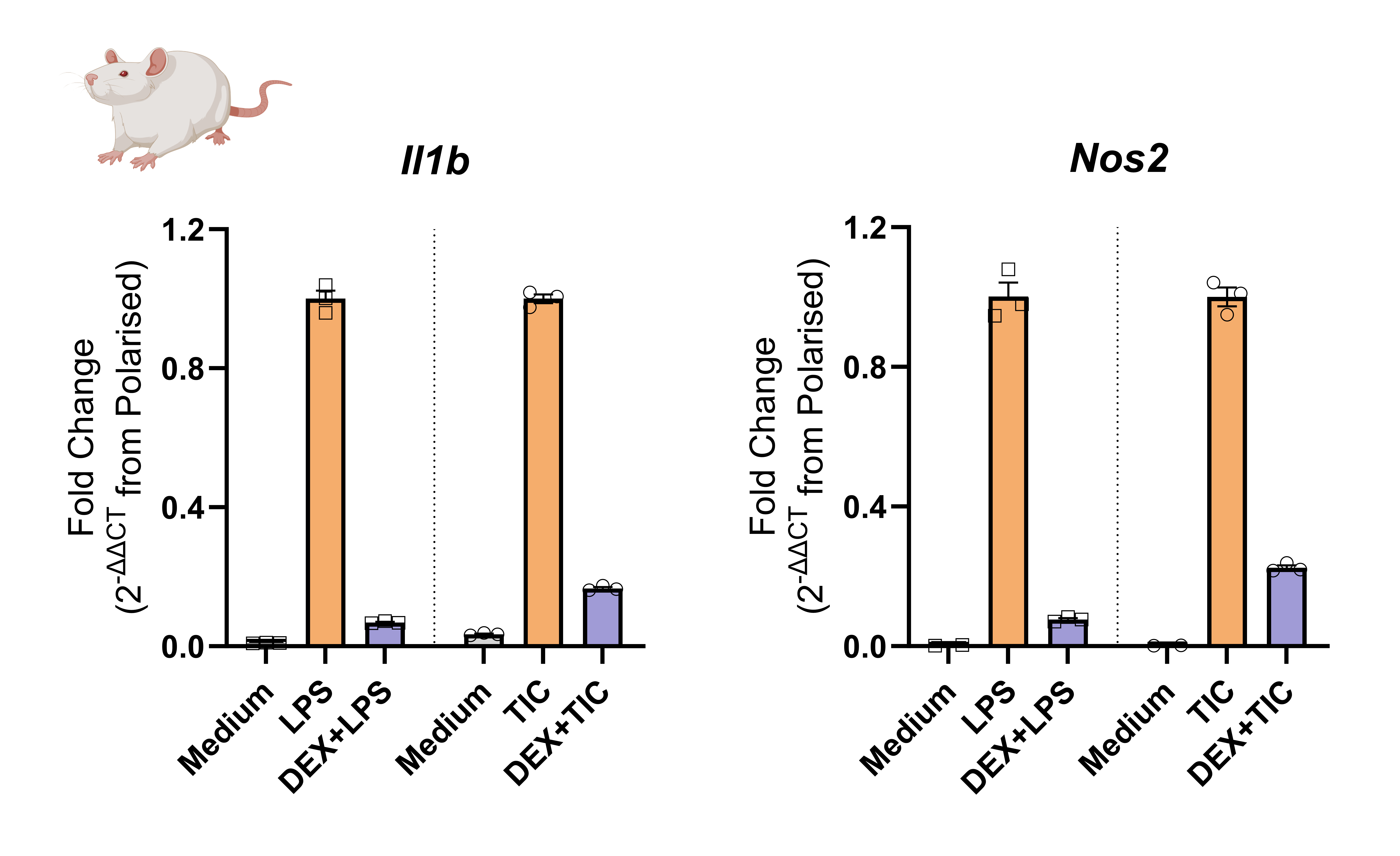
Figure 2. Following generation of mixed glial culture from neonatal Sprague Dawley (SD) rat brains, sequential shake-off of microglia and OPCs, astrocytes were isolated and plated into a 96-well plate. Human iPSC-derived astrocytes were thawed and plated into a 96-well plate. After a period of cell maturation, astrocytes were treated with Dexamethasone (Dex) and polarized with TNFα, IL-1αand C1q (TIC) or LPS. In these experiments, cytokine secretion profile was assessed via Luminex, gene expression was analyzed by RTqPCR.
Data analysis and results
Cytokine secretion profile
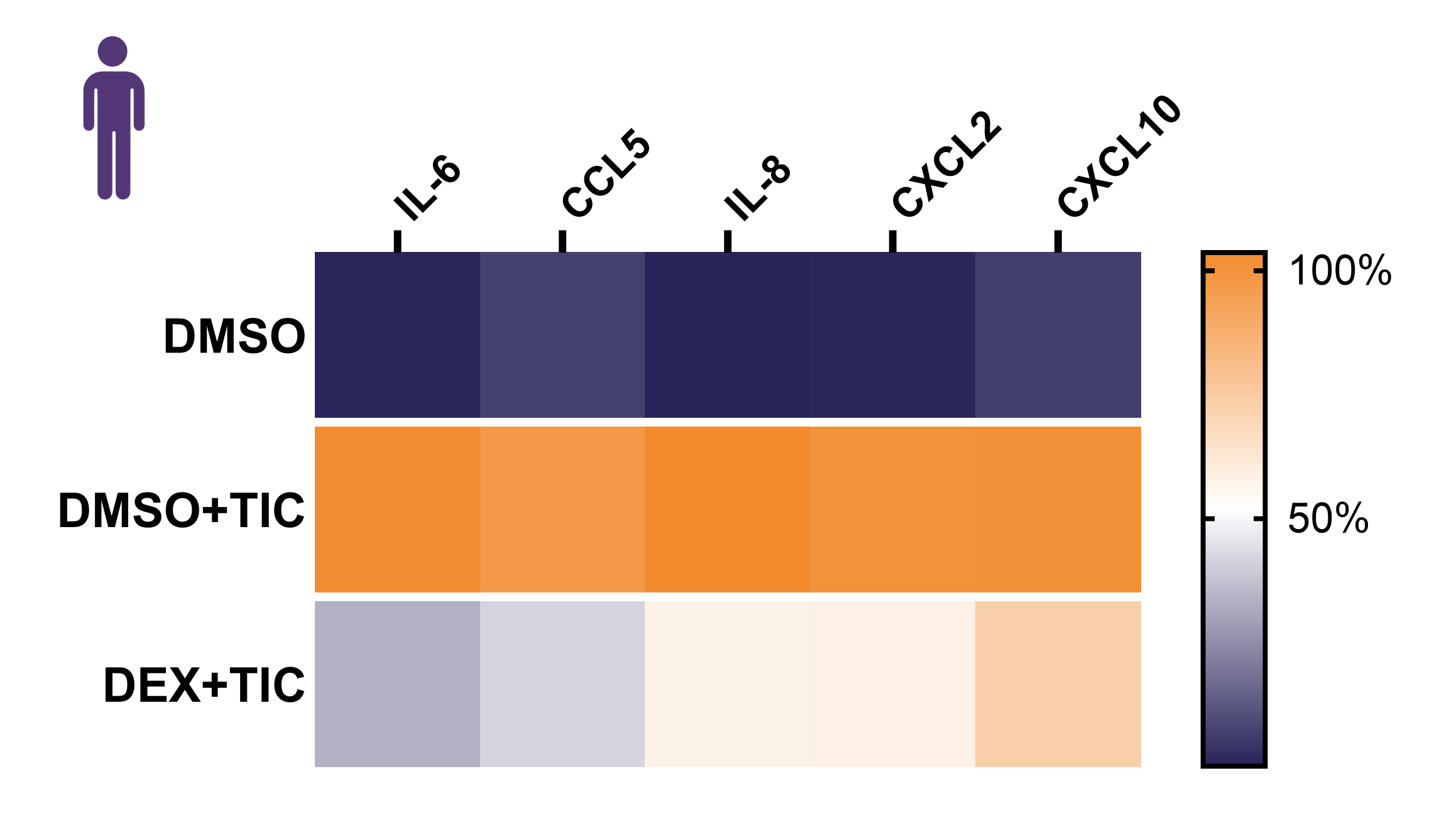
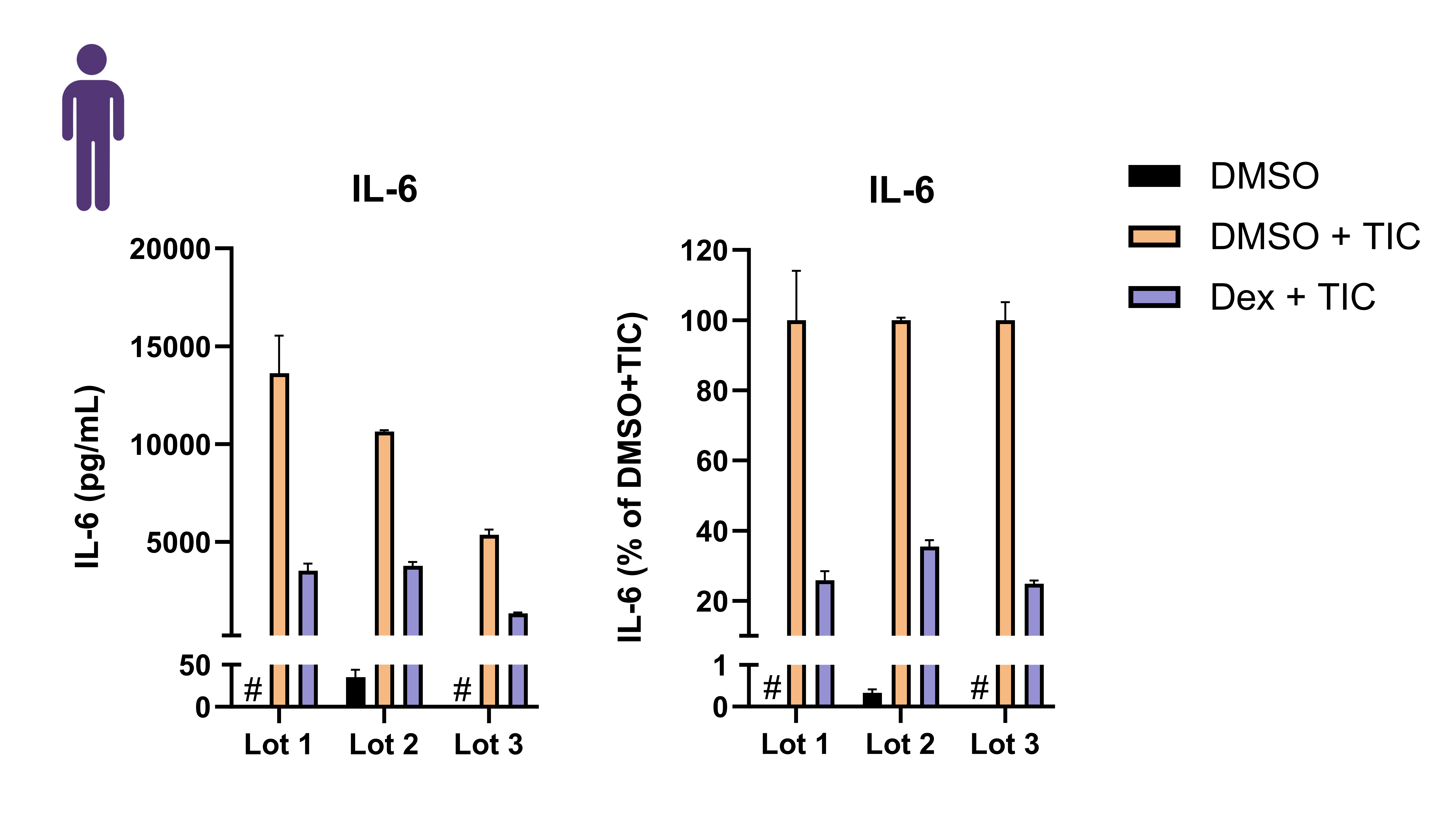
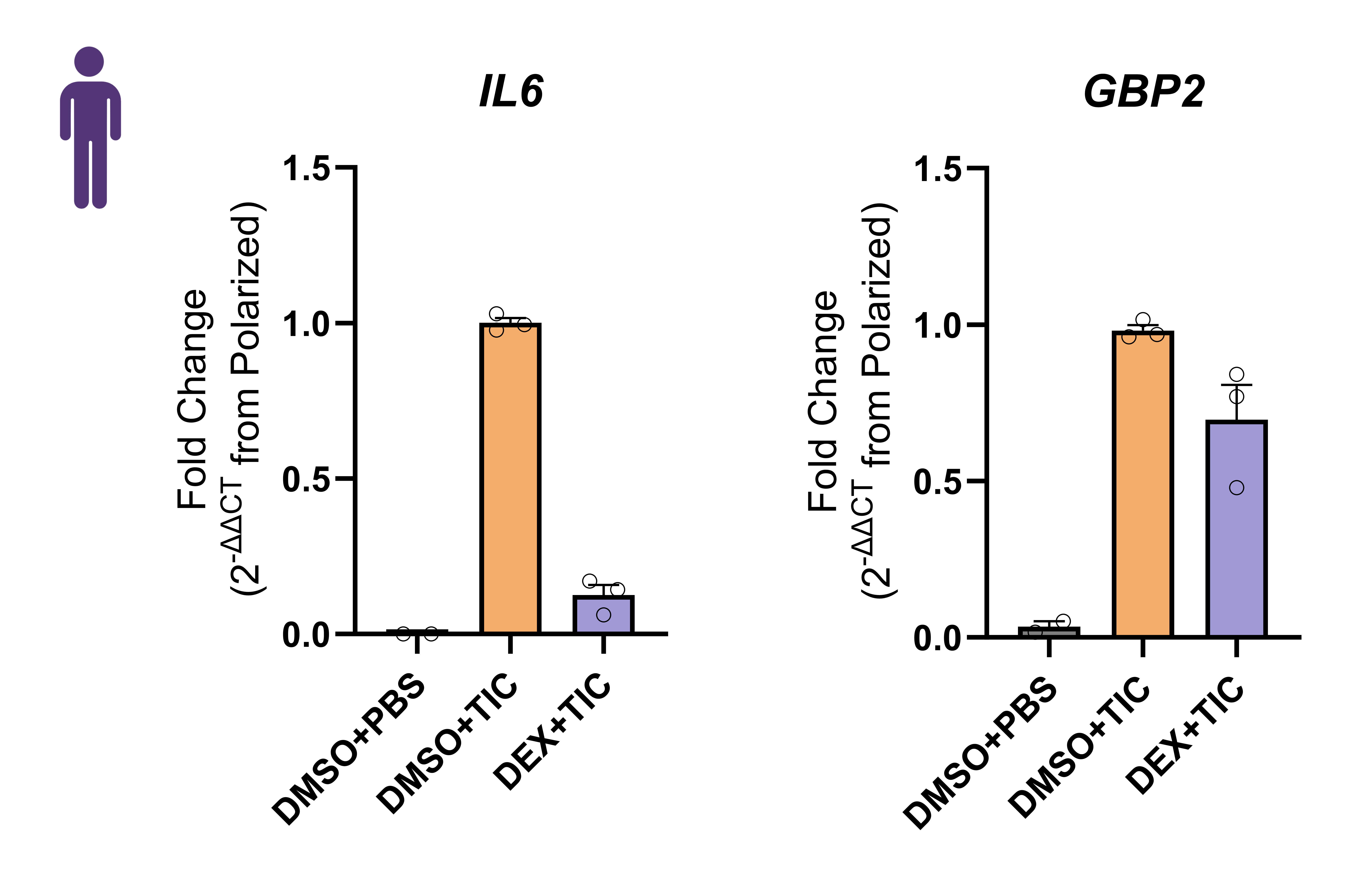
Conclusions
Astrocytic activation is both a consequence and a driver of chronic neuroinflammation in neurodegenerative diseases. Reactive astrocytes perpetuate the cycle of neurotoxicity, exacerbating neuronal damage in conditions such as Alzheimer’s and Parkinson’s disease.
This assay reliably models astrocyte polarization in both rodent and human systems, providing a robust platform for testing compounds with potential anti-inflammatory or neuroprotective effects.
