Accelerating your API to Clinic

Project overview
The challenge
- Overcoming scale up challenges to accelerate progression to GMP and clinical trials.
Our solution
- A solution designed to derisk manufacturing and analytical activities ensuring reliable API supply.
Our impact
- Empowered the client to progress to clinical trials with complete confidence in their manufacturing process and regulatory compliance strategy.
The challenge
Rapid, Phase-Appropriate, Process Development – Discovery to GMP
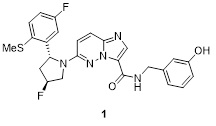
A client engaged us to support their medicinal chemistry program targeting a topical pan-Trk 1 inhibitor.
The initial synthetic route, though effective for early SAR exploration, presented significant challenges for scale-up. These included stereochemical control issues affecting yield and a highly inefficient critical fluorination step. Additionally, the use of hazardous reagents and formation of impurities increased regulatory burden, slowing down the project. The client needed a rapid, scalable, and regulatory-compliant process to move from discovery through to clinical trials—without compromising safety or quality.
Our approach
Integrated Process Development Excellence
Combining our cross-functional teams specializing in Discovery, PR&D, and GMP manufacturing, the strategic approach combined scientific oversight, flexible problem-solving, and advanced analytical capabilities. Early risk identification and mitigation ensured the route was both scalable and commercially viable. We applied a Design of Experiments (DoE) approach, resulting in high-quality API with well-characterized impurity profiles, exceeding regulatory requirements. Frequent client updates ensured alignment with expectations and timelines, enabling seamless technical transfer and accelerated development.
Our solution
Delivering Excellence Through Innovation
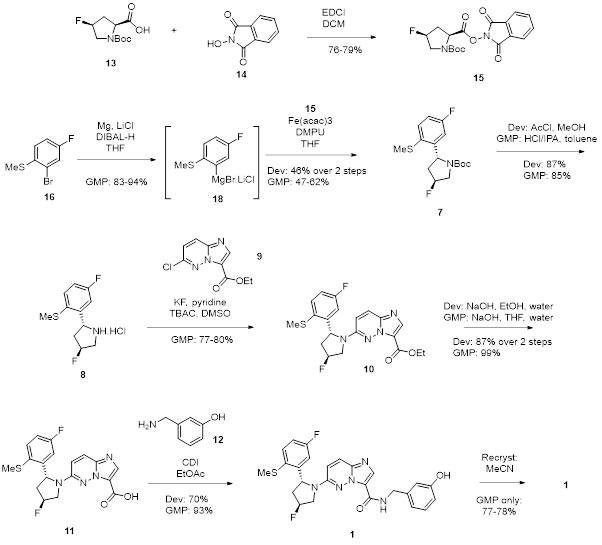
Through systematic route scouting and literature review, we redesigned the synthesis to introduce and control the relevant stereocentres with improved yield. Key developments included swapping nickel to iron catalysis allowed us to reduce toxicity and enhance yield. A robust recrystallization protocol was developed and the overall yield for the synthesis increased dramatically from 0.23% to 22%. Nearly 5 kg of high-purity API was manufactured, supported by validated analytical methods and full safety data.
The result
Measurable Success in Complex API Manufacturing
The integrated development strategy approach and partnership enabled the client to progress from hit-to-lead to clinical candidate nomination and beyond (i.e. clinic). The optimized, chromatography-free synthesis delivered high-quality API on time and in full at each stage, meeting the agreed quality specification and exceeding client expectations. This proactive approach to scale-up challenges and technology transfer ensured a smooth transition to GMP manufacturing. This empowered the client to progress to clinical trials with complete confidence in their manufacturing process and regulatory compliance strategy.
